
Higher and foundation tiers
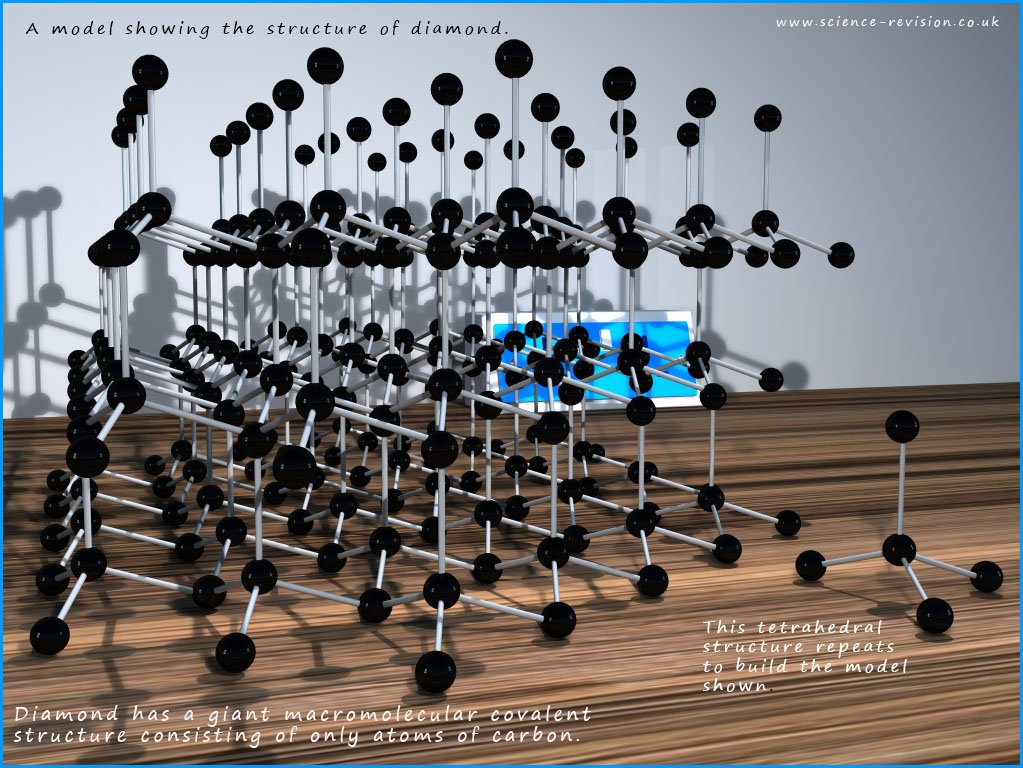
 What do diamonds and a pencil lead have in common? At first it may appear that they have very little in common.
Diamond
is the hardest substance known, it is an
electrical insulator, they are semi-transparent and they sparkle! Graphite on the
other hand is often used in pencil leads; it is a dull coloured material which is so
soft it can be used to write with
on paper. It is also slippery to the touch and is used as a
lubricant in industry. It is also a good electrical
and thermal conductor. It is also worth mentioning that
diamond is also an excellent
thermal conductor.
What do diamonds and a pencil lead have in common? At first it may appear that they have very little in common.
Diamond
is the hardest substance known, it is an
electrical insulator, they are semi-transparent and they sparkle! Graphite on the
other hand is often used in pencil leads; it is a dull coloured material which is so
soft it can be used to write with
on paper. It is also slippery to the touch and is used as a
lubricant in industry. It is also a good electrical
and thermal conductor. It is also worth mentioning that
diamond is also an excellent
thermal conductor.
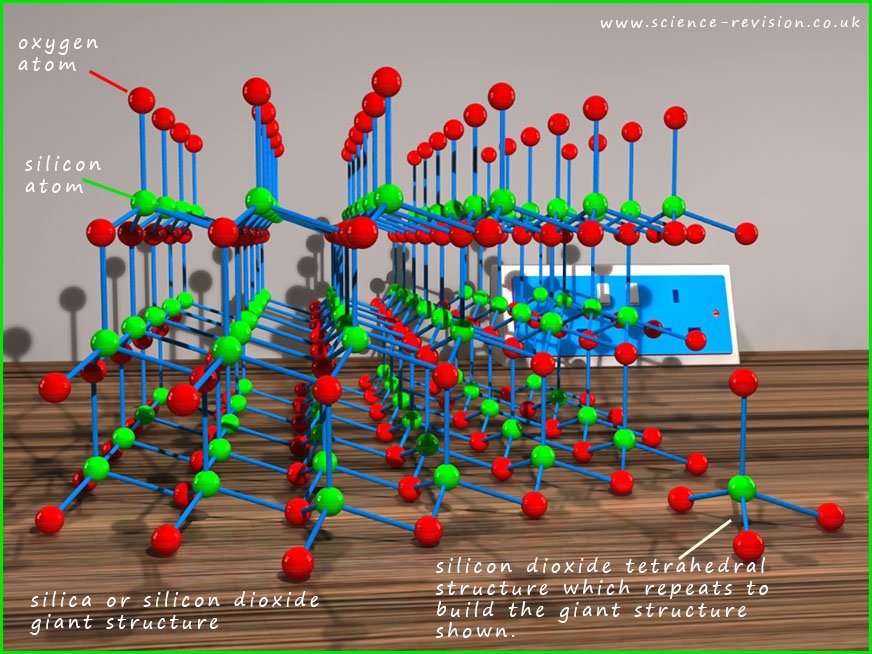
Silicon is underneath carbon in group four of the periodic table. You may notice lots of similarities between the structure of silicon dioxide (sand) shown below and that of diamond shown above. In the image below the green spheres are silicon atoms and the red spheres represent oxygen atoms. The silicon atoms form a tetrahedral structure with four oxygen atoms around a central silicon atom, this small tetrahedral shaped molecule is shown sitting in front of the giant covalent structure in the image above. This tetrahedral shape repeats to build up the giant silicon dioxide macromolecular structure. Silicon forms strong covalent bonds with oxygen, so silicon dioxide having a giant structure with lots of strong bonds will like diamond have a high melting and boiling point. There are also no free delocalised electrons present in silicon dioxide since all the valency electrons from the silicon and oxygen atoms are held tightly in the covalent bonds within the giant structure and so it is an electrical insulator. The image below shows the structure of silica or silicon dioxide.
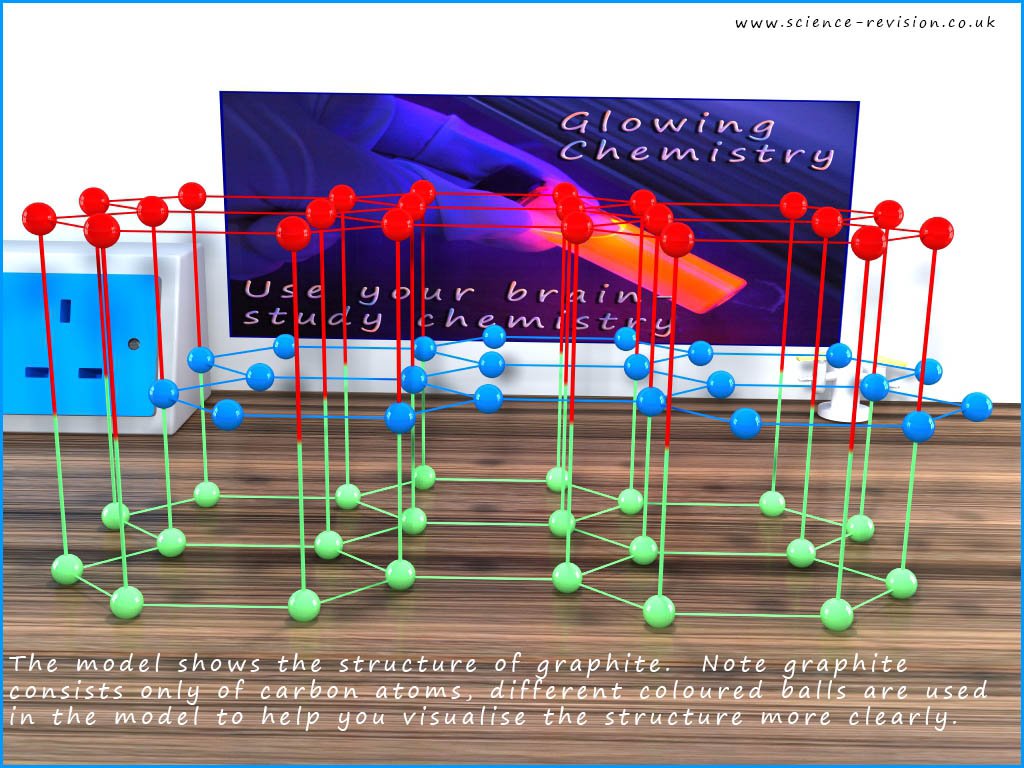
Graphite like diamond has a giant macromolecular structure but the carbon atoms in graphite are arranged in a very different way to that found in diamond. The image below shows how the carbon atoms are arranged in graphite. The carbon atoms in the image are coloured to help you visualize the structure of graphite. The carbon atoms in graphite are arranged in flat layers of hexagons, shown in green, blue and red in the image below.
By studying the picture you can see that each carbon atom makes only three covalent bonds, unlike the four covalent bonds each carbon atom makes in diamond. Since each carbon atom should make four bonds, this means that there are free or delocalised electrons within the structure. These free or delocalised electrons are found between the flat layers of hexagons and being delocalised means that graphite is a good electrical and thermal conductor.
 The flat layers of carbon atoms in the hexagon structure
are held together by strong covalent bonds between the carbon atoms;
however
there are only very weak intermolecular bonds (Van der Waals bonds)
between the separate layers of hexagons. This means that if
a small force is applied to one layer it can easily separate and slide free from the other layers.
This is one of the
main reasons why graphite is soft. When you write with a pencil
the force that you push down on the tip with is big
enough to break the weak intermolecular bonds between the hexagon layers and leave behind a
layer of flat hexagons on the paper. If you
scribble on paper over and over again
to leave a thick pencil mark then rub your
finger over the thick mark it will feel very slippery. This is because the
flat layers of hexagons left on
the paper are able to slide over each other and act as a lubricant.
The flat layers of carbon atoms in the hexagon structure
are held together by strong covalent bonds between the carbon atoms;
however
there are only very weak intermolecular bonds (Van der Waals bonds)
between the separate layers of hexagons. This means that if
a small force is applied to one layer it can easily separate and slide free from the other layers.
This is one of the
main reasons why graphite is soft. When you write with a pencil
the force that you push down on the tip with is big
enough to break the weak intermolecular bonds between the hexagon layers and leave behind a
layer of flat hexagons on the paper. If you
scribble on paper over and over again
to leave a thick pencil mark then rub your
finger over the thick mark it will feel very slippery. This is because the
flat layers of hexagons left on
the paper are able to slide over each other and act as a lubricant.